This file is a part of the Rhodium site archive. This Aug 2004 static snapshot is hosted by Erowid
as of May 2005 and is not being updated. > > Back to Rhodium Archive Index > >
as of May 2005 and is not being updated. > > Back to Rhodium Archive Index > >
A Modified Clemmensen Reduction Procedure for Conversion of Aryl Ketones Into Aryl Alkenes
G. A. Hiegel & J. R. Carney
Synth. Commun. 26, 2625-31 (1996)
HTML by RhodiumAbstract
Aryl alkenes can be prepared from aryl ketones through reduction by refluxing with amalgamated zinc in a mixture of formic acid and ethanol.Equation 1
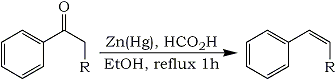
Our modification of the Clemmensen procedure which gives the highest ratio of alkene to alkane and the highest isolated yields involves refluxing the ketone with amalgamated zinc in 88% formic acid and 95% ethanol for 1 h. Significant results are summarized in the Table. Acetic acid could be used in place of formic acid, but replacement with phosphoric acid, ammonium chloride, or anilinium chloride resulted in a lower ratio of alkene or a very slow reaction. Methanol as the cosolvent was about as effective as ethanol, but cyclohexane, benzene, tert-butyl alcohol, dioxane, or no cosolvent gave a slightly lower ratio of alkene. Zinc amalgamated in the usual manner was used for most experiments and gave the best results. When methylene chloride was used for extraction during the work-up instead of diethyl ether, the isolated yields were improved significantly.2
1-Phenyl-2-propanone and 2-octanone, both alkyl ketones, did not react appreciably under the mild conditions used for aromatic ketones. 1-Phenyl-1-ethanone was reduced to a 16:84 GC ratio of ethylbenzene to styrene, but a distilled yield of only 19% was obtained. A 1H NMR of an undistilled sample showed the presence of a cyclopropyl group which is believed to be formed from a zinc-carbene intermediate (Zn=C) reacting with alkene.12
Rearrangement of the same zinc-carbene intermediate accounts for the formation of alkene. Under the milder Clemmensen reduction conditions used here, apparently aryl ketones easily form the zinc-carbene intermediate, and rearrangement to the alkene is normally the major reaction pathway.
Experimental
Reduction of 1-Phenyl-1-butanoneThis procedure was used for all reactions reported in the Table. In a round bottom flask were placed 10.11 g (0.155 mol) of Zn dust, 0.498 g (1.84 mmol) of HgCl2, 10 mL of H2O, 0.5 mL of conc. HCl, and a stir bar. After 10 min of stirring, the liquid was decanted, and any lumps were broken up with a spatula. To the flask were added 20 mL of 88% formic acid, 6 mL of 95% ethanol, and 2.0 mL (13.78 mmol) of 1-phenyl-1-butanone. The mixture was refluxed and stirred for 1 h. The liquid was decanted into a separatory funnel, and the residue stirred with 20 mL of H2O for 5 min. The water was also added to the separatory funnel and the residue stirred with 20 mL of CH2Cl2 for 5 min. The CH2Cl2 was used to extract the aqueous phase. The residue was washed two more times with 20 mL each of CH2Cl2, and the CH2Cl2 was used to extract the aqueous phase each time. The combined CH2Cl2 solution was washed with 1 N NaOH until the wash was basic (60 mL and 30 mL) and with sat. NaCl solution (60 mL) and dried over MgSO4. After filtration and solvent removal, the residue was distilled using a Kugelrohr apparatus to give 1.46 g of product (80% yield based on the molecular weight of the alkene) (bath temp 113-121°C at 43 torr).
The IR and NMR spectra were a good match for that of trans-1-phenyl-1-butene, and the NMR spectrum also showed peaks indicative of the presence of small amounts of cis-1-phenyl-1-butene, butylbenzene, and CH2Cl2. Analysis by GC (10% Carbowax 20M) showed significant peaks at 0.54 min (1.3%), 1.7 min (7.2%), 2.3 min (10.1%) 3.4 min (80.5%). The peaks at 0.54, 1.7, and 3.4 min correspond to the retention times of CH2Cl2, butylbenzene, and trans-1-phenyl-1-butene, respectively. The peaks at 2.3 and 3.4 min were collected by preparative GC. The NMR of the peak at 2.3 min agreed with that reported for cis-1-phenyl-1-butene,13 and the NMR of the peak at 3.4 min matched that of trans-1-phenyl-1-butene.
References
-
- Clemmensen, E., Chem. Ber. 1913, 46, 1837
- Clemmensen, E., Chem. Ber. 1914, 47, 51
- Clemmensen, E., Chem. Ber. 1914, 47, 681
- Martin, E.L., Org. Reactions, 1942, 1, 155.
- Staschewski, D., Angew. Chem., 1959, 71, 726.
- Vedejs, E., Org. Reactions, 1975, 22, 401.
- Brewster, J.H., J. Am. Chem. Soc., 1954, 76, 6364.
- Brewster, J.H., Patterson, J. and Fidler, D.A., J. Am. Chem. Soc., 1954, 76, 6368.
- Nakabayashi, T., J. Am. Chem. Soc., 1960, 82, 3906; see also 3900 and 3909.
- Risinger, G.E., Mach, E.E. and Barnett, K.W., Chem. Ind. (London), 1965, 679.
- Steinkopf, W. and Wolfram, A., Liebigs Ann. Chem., 1923, 430, 113.
- Motherwell, W.B., J. Chem. Soc. Chem. Commun., 1973, 935.
- Risinger, G.E. and Thompson, J.A., J. Appl. Chem., 1963, 13, 346.
- Mechanistic proposals for the Clemmensen reduction are found in references 3-5 and 7, but the explanation that best fits our data is found in Burdon, J. and Price, R.C., J. Chem. Soc. Chem. Commun., 1986, 893.
- Underwood, G.M., Chan, A.K., Green, T., Watts, C.T. and Kingsbury, C.A., J. Org. Chem., 1973, 38, 2735.
- Kikukawa, K., Sakai, K., Asada, K. and Matsuda, T., J. Organomet. Chem. , 1974, 77, 131.
- Tomioka, H, Tabayashi, K., Ozaki, Y. and Izawa, Y., Tetrahedron, 1985, 41, 1435.
- Newsoroff, G.P. and Sternhell, S., Aust. J. Chem., 1966, 19, 1667.
- Schaefer, T., Penner, G. H.,-Takeuchi, C.S., and Beaulieu, C., Can. J. Chem., 1989, 67, 1283.

Žiadne komentáre:
Zverejnenie komentára